Hydrothermal stability of cobalt silica membranes in a water gas shift membrane reactor
Dec 30, 2008·
,
 ,
,
,
,
·
0 min read
,
,
,
,
·
0 min read
S. Battersby
S. Smart
Prof. Dr. Bradley P. Ladewig
S. Liu
M. C. Duke
V. Rudolph
J. C. D. D Costa
Abstract
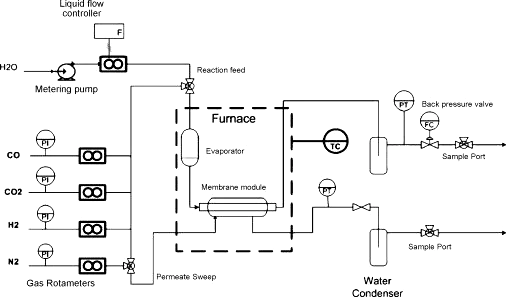
Cobalt silica membranes were fabricated using sol–gel techniques for separation of H2 in a membrane reactor set up for the low temperature (up to 300 °C) water gas shift (WGS) reaction. Single dry gas testing prior to reaction showed He/N2 and H2/CO2 selectivities increasing from 75–400 to 45–160 as the temperature increased from 100 to 250 °C, respectively. During reaction the membrane delivered a H2 permeation purity of 89–95% at high conversions, with the higher water ratio conversion providing superior membrane operational performance. Characterisation of bulk gels indicated that the cobalt silica was hydrophilic and exposure to steam at 200 °C resulted in the densification of the film matrix. The cobalt doping allowed for the membrane structural microporosity to be maintained as H2 selectivity was not affected by steam exposure, though the flux decreased due to pore collapse of the film matrix. A total of 8 thermal cycle testing were carried out from room temperature to 300 °C, and the membrane displayed good hydrothermal stability, maintaining a high H2 selectivity for over 200 h of operation.
Type
Publication
Separation and Purification Technology