Can metal organic frameworks outperform adsorptive removal of harmful phenolic compound 2-chlorophenol by activated carbon?
Apr 1, 2020·
,
 ·
0 min read
·
0 min read
L. H. Mohd Azmi
D. Williams
Prof. Dr. Bradley P. Ladewig
Abstract
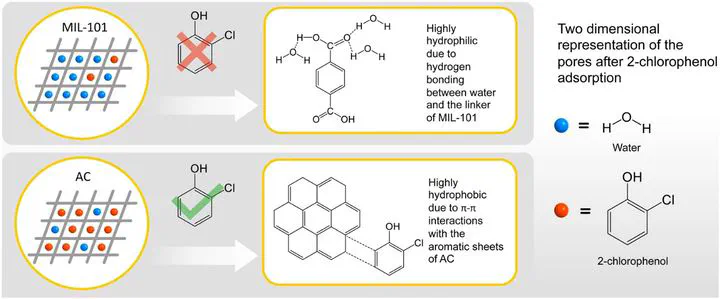
Removal of persistent organic compounds from aqueous solutions is generally achieved using adsorbent like activated carbon (AC) but it suffers from limited adsorption capacity due to low surface area. This paper describes a pioneering work on the adsorption of an organic pollutant, 2-chlorophenol (2-CP) by two MOFs with high surface area and water stability; MIL-101 and its amino-derivative, MIL-101-NH2. Although MOFs have higher surface area than AC, the latter was proven better having the highest equilibrium 2-CP uptake (345 mg g−1), followed by MIL-101 (121 mg g−1) and MIL-101-NH2 (84 mg g−1). Used MIL-101 could be easily regenerated multiple times by washing with ethanol and even showed improved adsorption capacity after each washing cycle. These results can open the doors to meticulous adsorbent selection for treating 2-CP-contaminated water.
Type
Publication
Chemical Engineering Research and Design