Extraction of the intrinsic rate constant for a photocyclization reaction in capillary microreactors using a simplified reactor model
May 9, 2024·
,
 ,
,
,
·
0 min read
,
,
,
·
0 min read
J. Li
H. Šimek Tosino
Prof. Dr. Bradley P. Ladewig
N. Jung
S. Bräse
R. Dittmeyer
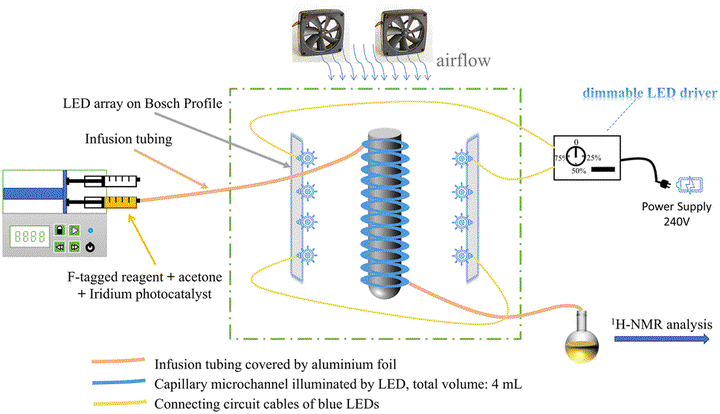 Schematic diagram of the continuous flow test bench for the photocyclization of the F-tagged aniline derivative to the indole product.
Schematic diagram of the continuous flow test bench for the photocyclization of the F-tagged aniline derivative to the indole product.
Abstract
In this work, a simple reactor model for evaluating the intrinsic rate constant of a photocyclization reaction is presented. The photoreaction was performed in a standardized capillary microreactor that ensures isothermal and uniform irradiation conditions. The effects of residence time and incident light intensity on the reaction performance were studied, and a reaction kinetic model was established based on a plug flow assumption. The reaction order with respect to the F-tagged amide precursor was found to be 2 in the photochemical transformation, and apparent rate constants under various light intensities were obtained. Comprehensive mass transport diagnostics were performed by using dimensionless numbers based on the established effective reaction kinetics. The intrinsic rate constant of the photoreaction was extracted from the experimental data using a simplified reactor model, in which a parameter representing the photon absorption fraction of the photocatalyst was introduced. Moreover, the proposed reactor model gives a general overview for improving the space-time yield of photochemical processes in microreactors. © 2024 The Royal Society of Chemistry.
Type
Publication
Reaction Chemistry and Engineering