Functional role of B-site substitution on the reactivity of CaMFeO3 (M = Cu, Mo, Co) perovskite catalysts in heterogeneous Fenton-like degradation of organic pollutant
Jan 1, 2023·
,
,
 ,
,
,
,
·
0 min read
,
,
,
,
·
0 min read
R. Alrozi
N. A. Zubir
N. F. A. Bakar
Prof. Dr. Bradley P. Ladewig
J. Motuzas
N. H. H. A. Bakar
D. K. Wang
J. C. D. Da Costa
Abstract
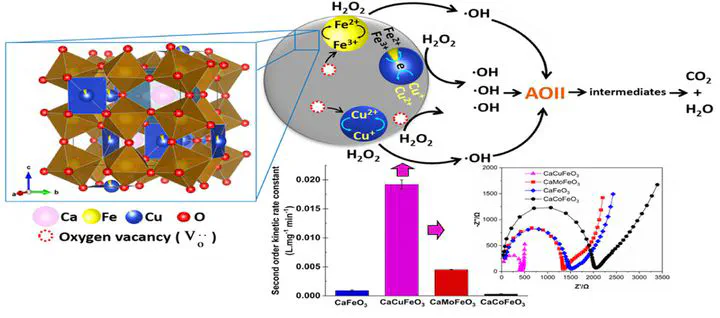
Background: Substitution of different types of B-site metal cations in the perovskite structure led to a significant change in the catalytic reactivity of the resulting catalysts. In this work, the functional role of B-site substitution on the catalytic reactivity of mixed oxides containing B-site substituted CaMFeO3 (M = Cu, Mo and Co) perovskite catalysts is investigated. Methods: The catalysts were synthesized via a modified EDTA-citric acid complexation method and tested for the heterogeneous Fenton-like reaction for oxidative degradation of acid orange II (AOII) dye in the presence of H2O2. Significant findings: CaCuFeO3 exhibited the highest AOII degradation (97%) followed by CaMoFeO3 (90%), CaFeO3 (64%) and CaCoFeO3 (40%) within 60 min of reaction, and the reaction followed a pseudo-second-order kinetics model. Interestingly, the partial substitution of Cu in the B-site of CaFeO3 enhanced the reaction rate constant achieving a k value of 1.9 ×10− 2 L mg− 1 min− 1 , approximately twenty-one times higher than that of the blank catalyst CaFeO3. The enhanced catalytic reactivity of CaCuFeO3 is associated with the high reducibility of copper/iron ions within the B-site structure in the presence of oxidant which facilitated fast redox cycling of the active sites during catalysis. The fast redox cycling is attributed to the decent electron mobility due to low electron transfer resistance between the active sites.
Type
Publication
Journal of the Taiwan Institute of Chemical Engineers