Nafion membranes modified by cationic cyclodextrin derivatives for enantioselective separation
Mar 10, 2021·
,
,
 ,
,
,
·
0 min read
,
,
,
·
0 min read
J. Gaálová
M. Michel
M. Bourassi
Prof. Dr. Bradley P. Ladewig
P. Kasal
J. Jindřich
P. Izák
Abstract
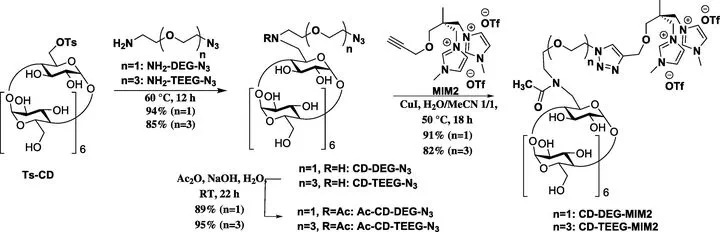
Nafion117® membranes modified by three cationic cyclodextrin (CD) derivatives have been prepared by strong ionic bonding. All CD derivatives contained bis(methylimidazolium) (MIM2) cationic anchor covalently bound to the CD unit, either using no spacer or using diethylene glycol (DEG) or tetraethylene glycol (TEEG) spacers. The modified membranes were tested in chiral separation of a model racemic mixture (d/l-tryptophan) from water. Different experimental set-ups for characterising membranes in enantioselective separation – pertraction, two kinds of sorption, and pressure-driven membrane separation – have been described and rigorously compared. The membranes CD-MIM2, CD-DEG-MIM2 have reached the highest enantiomeric excess, 14 and 44% respectively, in 280 days. The lowest performance of the CD-TEEG-MIM2 membrane, with the long spacer, has been visibly ameliorated by applying pertraction; enantiomeric excess rose from 2 to 27% in 80 days. Even though sorption played the main role in pertraction, this process substantially enhanced the separation of racemic mixtures. The pressure-driven approach has allowed the operation to be continuous and faster, which has the potential for continuous large-scale production of enantiopure compounds and could pave the way for many more commercial applications, satisfying the considerable demand for large-scale chiral separation techniques.
Type
Publication
Separation and Purification Technology