Systematic screening of DMOF-1 with NH2, NO2, Br and azobenzene functionalities for elucidation of carbon dioxide and nitrogen separation properties
Abstract
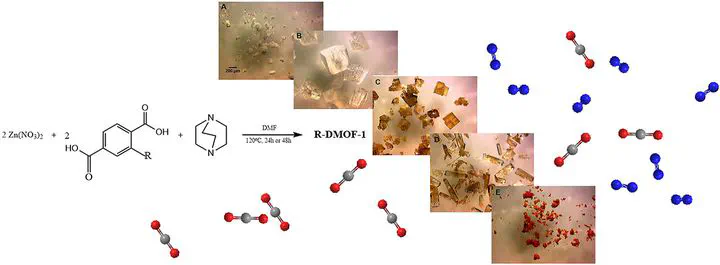
In this study, dabco MOF-1 (DMOF-1) with four different functional groups (NH2, NO2, Br and azobenzene) has been successfully synthesized through systematic control of the synthesis conditions. The functionalised DMOF-1 is characterized using various analytical techniques including PXRD, TGA and N2 sorption. The effect of the various functional groups on the performance of the MOFs for post-combustion CO2 capture is evaluated. DMOF-1s with polar functional groups are found to have better affinity with CO2 compared with the parent framework as indicated by higher CO2 heat of adsorption. However, imparting steric hindrance to the framework as in Azo-DMOF-1 enhances CO2/N2 selectivity, potentially as a result of lower N2 affinity for the framework.
Type
Publication
Inorganic Chemistry Communications