Effect of carbonization temperature on adsorption property of ZIF-8 derived nanoporous carbon for water treatment
Aug 22, 2016·
,
,
 ,
,
·
0 min read
,
,
·
0 min read
Z. Abbasi
E. Shamsaei
S. K. Leong
Prof. Dr. Bradley P. Ladewig
X. Zhang
H. Wang
Abstract
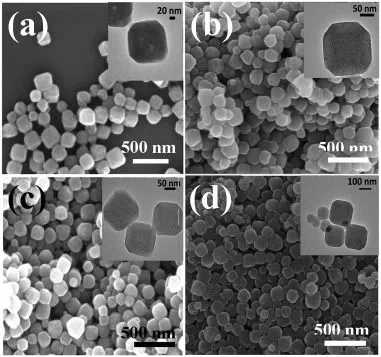
The heat treatment effect on the adsorption capabilities of nanoporous carbon particles derived from Zeolitic Imidazolate Framework-8 (ZIF-8) was investigated at 600, 1000 and 1200°C in this study. The results showed that heat treatment at 1000°C had a significant effect on the adsorption capacity of ZIF-8 (almost 10 times) for the removal of methylene blue (MB) dye from water. Nanoporous carbons were synthesized by direct carbonization of ZIF-8. SEM and TEM images showed that the carbon resulting from ZIF-8 carbonization at various temperatures retained the original structure and morphology of ZIF-8. The carbon nanoparticles carbonized at 1000°C exhibited outstanding adsorption capacities (186.3 mg/g) compared to nanoparticles carbonized at 600°C (49.5 mg/g) and 1200°C (36.7 mg/g) as well as ZIF-8 (19.5 mg/g) due to the change in surface charge and pore size distribution. The surface functionalities of materials were also characterized by Raman Spectroscopy, N2 adsorption-desorption, FTIR and TGA. The surface charge of the carbon particles changed from positive (ZIF-8) to negative as a result of conversion to carbon confirmed by zeta potential of the samples. The ZIF-8 derived carbon nanoparticles were found to be efficient adsorbents for water treatment purposes due to the satisfactory adsorption properties such as high adsorption capacity and good wettability.
Type
Publication
Microporous and Mesoporous Materials